Introduction to the Accelerated Approval Pathway

The FDA has developed several mechanisms to speed drugs to market when a compelling medical need exists including Accelerated Approval (AA), Priority Review, Fast Track, and Breakthrough Therapy Designation. Accelerated Approval is an important regulatory pathway that provides patients with earlier access to treatments for serious medical conditions when there is an unmet medical need. This is the first post in a 3‑part series on the AA Pathway. Herein, I’ll focus on how this pathway is used to bring drugs to market and highlight some of the key issues surrounding it.
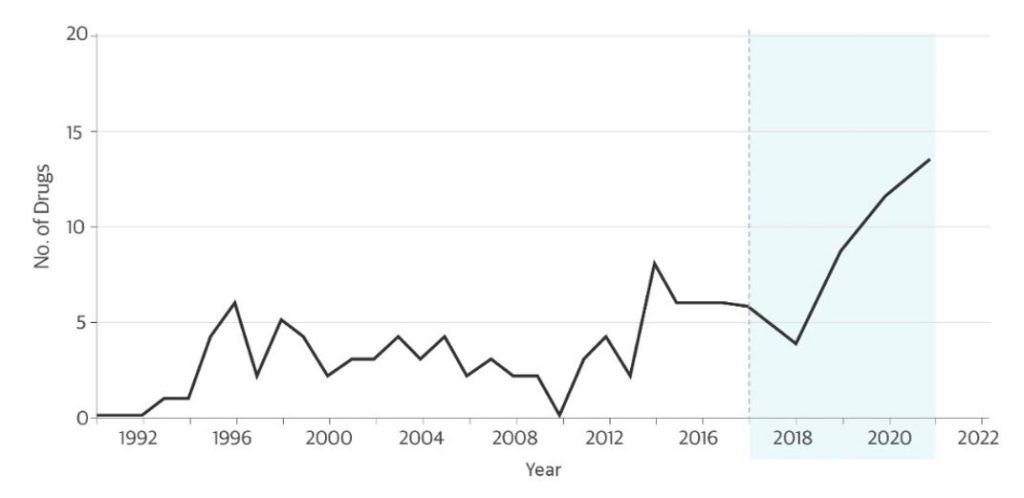
The AA Pathway was created in 1992 in response to the AIDS crisis and was codified in 2012 under the Food and Drug Administration Safety Innovations Act (FDASIA) in an effort to bring potentially life-saving drugs to market more quickly. The majority of drugs granted AA have been for AIDS, rare diseases, and cancer. Over the past 10 years, there has been a steady increase in the number of New Drug Applications (NDAs) seeking AA (Figure1,2); this increase has been driven largely by immune checkpoint inhibitors, which have transformed the treatment of many types of cancer.
Figure: Novel Drugs Approved Using the Accelerated Approval Pathway.
Data sources: Darrow JJ, et al. JAMA. 2020 (through 2017);1 FDA CDER reports 2017-20212
How does Accelerated Approval work?
Under normal or traditional FDA approval pathways, a drug must show evidence of clinical benefit based on endpoints that measure how a patient feels, functions, or survives. In contrast, AA allows FDA to grant a drug a “conditional” approval based on its effect on a surrogate or an intermediate clinical endpoint that is reasonably likely to predict clinical benefit.3 (We’ll share more on this in part 2 of this series.) In some cases, AA can shave years off the clinical development timeline. Under the same law, the FDA requires sponsors to conduct a confirmatory clinical trial in the postmarketing setting to confirm that the drug does provide meaningful clinical benefit, and FDA can withdraw approval if further trials fail to verify the predicted clinical benefit.
Pros of the Accelerated Approval Pathway
The majority of drugs granted AA are for rare diseases, where the unmet need is great (90% of rare diseases have no approved therapy),4 and for oncologic indications where the disease is often life-threatening. In the case of rare diseases where patients are few and far between, it can be extremely challenging to conduct controlled clinical trials of sufficient size to demonstrate clinical benefit using traditional endpoints. The use of surrogate endpoints under an AA may be the only feasible path to approval; however, it can often be difficult subsequently to confirm clinical benefit in the postmarketing setting. In the case of oncology drugs, clinicians want to have access to promising new therapies for patients who have limited time to wait for the latest, potentially life-saving innovation, so speed to market becomes paramount.
Cons of the Accelerated Approval Pathway
The nature of the AA Pathway allows drugs to be marketed for a given indication before clinical benefit has actually been demonstrated using validated clinical endpoints. Although a surrogate endpoint must be reasonably likely to predict clinical benefit, this anticipated benefit must be verified in a confirmatory trial.
FDA prefers that a well-designed confirmatory trial be planned or underway at the time of AA, but there is no action enforceable by the FDA to require completion of confirmatory trials. According to a recent investigation in the British Medical Journal, nearly half of the drugs granted AA have not confirmed clinical benefit,5 and only 16 AAs have been withdrawn. This calls into question whether some of the drugs approved through the AA pathway are actually providing the promised clinical benefit to patients. That being said, there are many situations, particularly in rare diseases, where it can be difficult to complete confirmatory trials.
Finally, even more problematic, is what to do when confirmatory trials fail. In some cases, FDA will convene an advisory committee meeting to discuss whether the unmet need still exists and whether additional ongoing trials may confirm clinical benefit. (We’ll share more on this in part 3 of this series.)
Conclusion
While confirmatory trials can be difficult to complete, the AA pathway has been used to approve an increasing number of novel drugs in the past 10 years and remains an important regulatory pathway that provides patients with earlier access to treatments for serious medical conditions. Stay tuned for our next installment, which will explore the use of surrogate endpoints in Accelerated Approvals.
 Jackie Orabone, PhD, helps clients prepare for FDA Advisory Committee meetings by combining her scientific expertise and research knowledge in immunology with over 2 years of medical communications agency experience. Connect with Jackie on LinkedIn.
Jackie Orabone, PhD, helps clients prepare for FDA Advisory Committee meetings by combining her scientific expertise and research knowledge in immunology with over 2 years of medical communications agency experience. Connect with Jackie on LinkedIn.
References:
- Darrow JJ, Avorn J, Kesselheim, AS. FDA approval and regulation of pharmaceuticals, 1983-2018. JAMA. 2020;323(2):164-176.
- US FDA. New Drugs at FDA: CDER’s New Molecular Entities and New Therapeutic Biological Products. Current as of January 27, 2022. Accessed March 3, 2022. https://www.fda.gov/drugs/development-approval-process-drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products
- US FDA. Accelerated Approval. Current as of January 4, 2018. Accessed March 3, 2022. https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/accelerated-approval
- Huron J. New report finds medical treatments for rare diseases account for only 11% of US drug spending; nearly 80% of orphan products treat rare diseases exclusively. March 4, 2021; National Organization for Rare Disorders. Accessed March 3, 2022. https://rarediseases.org/new-report-finds-medical-treatments-for-rare-diseases-account-for-only-11-of-us-drug-spending-nearly-80-of-orphan-products-treat-rare-diseases-exclusively/#:~:text=Approximately%207%2C000%20known%20rare%20diseases,rare%20diseases%20have%20no%20treatment
- Mahase E. FDA allows drugs without proven clinical benefit to languish for years on accelerated pathway. BMJ. 2021;374:n1898.