Building a Better CAR: Emerging Technology and Safety Concerns Shake Up the Field
When chimeric antigen receptor (CAR)‑T cells first rolled off the assembly line 7 years ago, with the approval of Kymriah, they were the newest and most technically advanced immunotherapy against cancer, and they demonstrated the potential to elicit profound and durable responses in patients with relapsed/refractory hematologic malignancies. The patient’s own (autologous) T cells are genetically modified in the lab to express a CAR, which equips them to recognize and destroy tumor cells expressing the antigen.
But there was always a nagging concern that the vectors used to genetically engineer the patient’s T cells could integrate into the wrong site in the genome, resulting in insertional mutagenesis. Unfortunately, there were not enough data, until recently, to know just how great that risk might be. In addition, the production of autologous CAR‑T cells (ie, derived from the patient’s own cells) is an expensive, labor-intensive process that takes several weeks or even longer.
Fast forward 7 years, and we now have a variety of new, emerging technologies with the potential to overcome some of the challenges facing traditional CAR‑T cells. Biotech companies are engineering allogeneic CAR‑T cell lines, CAR-natural killer (NK) cells, and deploying an army of antibodies known as bispecific T-cell engagers (BiTEs) to do what autologous CAR‑T cells can do, with fewer downsides.
Risk of Insertional Mutagenesis With First-Generation CAR-T Cells
Since the beginning of the CAR‑T cell era, 6 products have been approved in the United States. These include 4 CD19‑directed therapies for B‑cell leukemia or lymphoma, which include tisa‑cel (Kymriah), axi‑cel (Yescarta), liso‑cel (Breyanzi), and brexu‑cel (Tecartus), along with 2 products that target B-cell maturation antigen for treating multiple myeloma — cilta‑cel (Carvykti) and ide‑cel (Abecma). According to a new FDA report, published in The New England Journal of Medicine (NEJM) by Nicole Verdun, MD, and Peter Marks, MD, PhD, from the FDA’s Center for Biologics Evaluation and Research, the risk of insertional mutagenesis leading to secondary cancers could be as high as about 1 in 1,000 (0.1%). To date, approximately 27,000 doses of these 6 CAR‑T cell products have been administered, and there have been 22 reported cases of secondary cancers, including at least 14 T‑cell malignancies.
Although it’s not possible to definitively prove that insertion of the CAR transgene caused all those secondary cancers, in 3 of 3 cases where the malignant clone has been sequenced, the CAR transgene was detected. This suggests that it’s certainly possible that insertion of the transgene caused the secondary malignancy. Although the risk is low and considered acceptable for an effective treatment for aggressive and often incurable hematologic malignancies, it remains a concern.
So much so that the FDA has called for a black box warning for all CAR‑T cell therapies. On January 19, 2024, the FDA issued letters to all the manufacturers and gave them 30 days to submit proposed changes to their safety labels or file a rebuttal. The FDA also reminded the manufacturers of their postmarketing requirement to conduct 15‑year observational safety studies to further investigate the long-term risk of secondary malignancies. In their NEJM perspective, Verdun and Marks suggest that new, more targeted, gene delivery strategies may help reduce the risk. That will be particularly critical as CAR‑T cell therapies are applied outside of oncology, including in autoimmune and infectious diseases.1
Indeed, research being conducted in Germany and recently reported in NEJM has shown that CD19-directed CAR‑T cells can reduce or eliminate the debilitating symptoms associated with a variety of autoimmune diseases, such as systemic lupus erythematosus, idiopathic inflammatory myositis, and systemic sclerosis. By depleting B cells, the researchers hope to essentially “reset” or “reboot” the cellular immune response and achieve lasting remission in patients with severe symptoms refractory to conventional therapies.
Allogeneic CAR‑T Cells — Next Generation Technology
Given the inherent limitations of producing autologous CAR‑T cells, including complexity and cost, the research community and biotech industry have leapt to the task of devising strategies to produce off-the-shelf allogeneic CAR‑T cells that can be mass produced and deployed quickly at lower cost. The two main challenges are preventing rejection and avoiding graft-versus-host disease (GVHD). In other words, the recipient’s immune system might reject the foreign cells, or the foreign T cells might attack the patient’s tissues and organs, resulting in GVHD.
To overcome these challenges, labs around the world are applying advanced gene editing technology to genetically modify allogeneic T cells.2 Those modifications are designed to ensure that the T cells will not be recognized and targeted as foreign by the recipient’s immune system, and conversely, that the T cells will not attack the recipient’s tissues. Disabling or replacing the endogenous T‑cell receptor and genes encoding HLA class I and/or class II can, in theory, create benevolent and stealthy allogeneic CAR‑T cells that can fly under the radar and persist in the patient long term. The trick will be to do that without disabling the functional or proliferative capacity of the T cells or making them targets for destruction by NK cells. A variety of advanced technologies are being explored to achieve that goal, including vectors that direct integration of the CAR gene construct into a specific genetic locus while at the same time disabling or replacing a gene of interest.
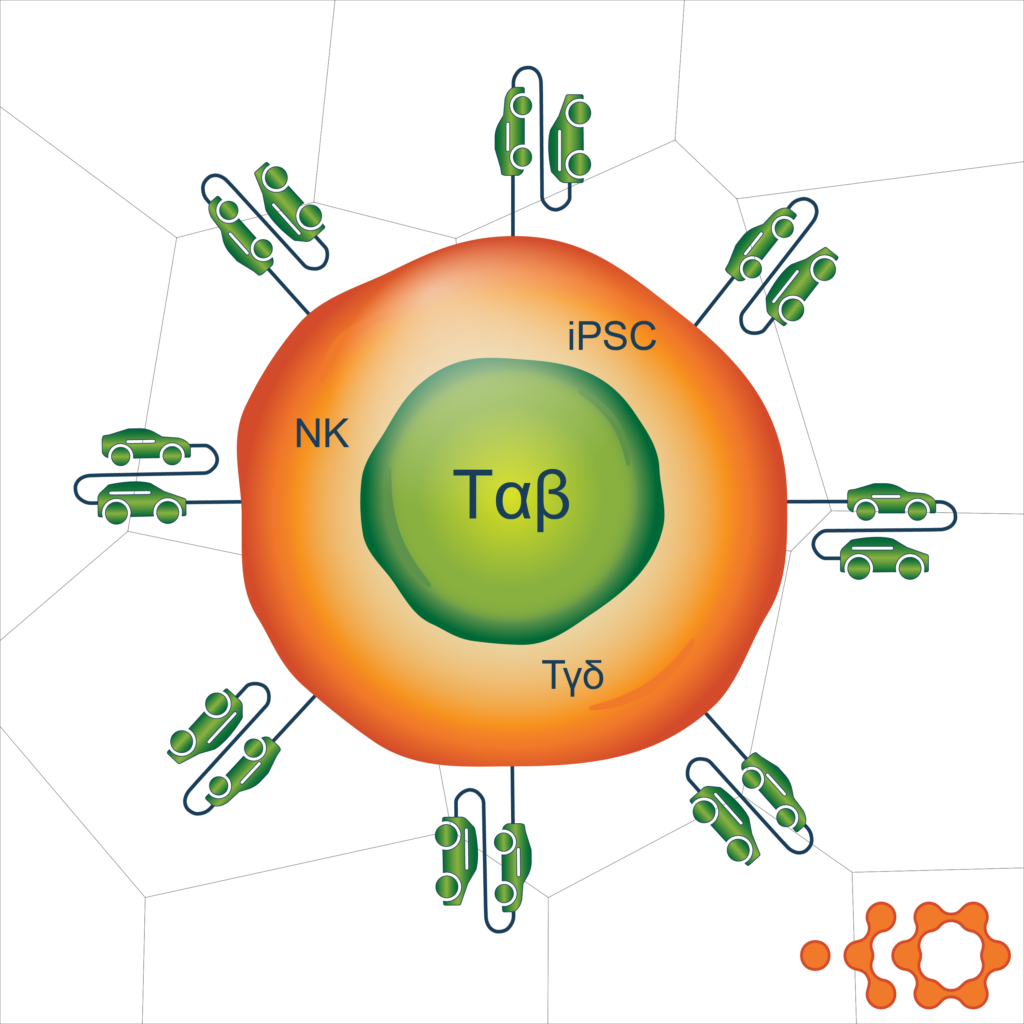
The type of allogeneic cells used to generate CAR-T cells is another area of innovation.2 One way to avoid GVHD is to use cell types other than the αβ T cells typically used to generate autologous CAR‑T cells. Options include γδ T cells, induced pluripotent stem cells (iPSC), hematopoietic stem cells derived from umbilical cord blood, memory T cells, virus-specific T cells, and cytokine-induced killer cells. These cell types may have various advantages in different therapeutic settings and can all be manipulated using different culture conditions to achieve the desired phenotype and maximize antitumor activity.
In addition, efforts are underway to harness the innate, HLA-independent, antitumor activity of NK cells.3 Natural killer cells can be harvested from peripheral or umbilical cord blood or iPSC and modified with constructs similar to those used for targeting and activation of CAR‑T cells. Clinical investigations are in early stages, and more work is needed to optimize this strategy, but progress is being made.
Bispecific T-cell Engagers (BiTEs)
Another strategy that is gaining traction is the use of BiTEs to empower the patient’s own T cells to attack the tumor more effectively. Bispecific antibodies are actually not new technology. They’ve been around for decades. But more recently, they’ve been engineered to bring T cells into close proximity with tumor cells. These antibodies are known as BiTEs. One arm of the antibody binds to a receptor on the surface of the T cell (most commonly CD3), and the other arm of the antibody binds to a molecule on the surface of the tumor cell. Similar to CAR‑T cells, this allows the patient’s T cells to attack and potentially kill the tumor cells, except that BiTEs are readily available (ie, off-the-shelf) and can be given to any patient without delay. 2023 was the year that BiTEs started to really take a bite out of the cancer immunotherapy market. We’ll have more on this topic in an upcoming blog post.
CAR‑T cell therapies have truly been transformational for patients with relapsed/refractory leukemia, lymphoma, or multiple myeloma, and solid tumors are the next frontier, but the inherent limitations of producing autologous CAR‑T cells remain a barrier. Next-generation cellular immunotherapies that can benefit many more patients are on the horizon, but will the biopharma industry be able to meet the demand? According to Pierre Luzeau, CEO of pharmaceutical manufacturing provider Seqens, “off-the-shelf CAR-T therapies are probably the future, but there is a requirement to simplify the manufacturing process to give these therapies a chance to expand massively in the next years.”
Jeff Riegel, PhD
SVP, Scientific Communications
Jeff combines his scientific expertise in molecular biology and immunology with more than 28 years of global healthcare agency experience guiding medical and regulatory communication strategies for biopharma companies. Jeff helps clients prepare for FDA Advisory Committee meetings and other health authority interactions. Connect with Jeff on LinkedIn.
References:
- Zmievskaya E, Valiullina A, Ganeeva I, et al. Application of CAR-T cell therapy beyond oncology: autoimmune diseases and viral infections. Biomedicines. 2021;9(1):59. Published online January 9, 2021. doi: 10.3390/biomedicines9010059
- Aparicio C, Acebal C, González-Vallinas M. Current approaches to develop “off-the-shelf” chimeric antigen receptor (CAR)-T cells for cancer treatment: a systematic review. Exp Hematol Oncol. 2023;12:73. Published online August 21, 2023. doi: 10.1186/s40164-023-00435-w.pdf.
- Moscarelli J, Zahavi D, Maynard R, Weiner LM. The next generation of cellular immunotherapy: CAR-NK cells. Transplant Cell Ther. 2022;28(10):650-656.