The Alzheimer’s Conundrum
The United States is facing an avalanche of Alzheimer’s disease (AD). An estimated 12.7 million Americans over the age of 65 are projected to suffer from AD dementia by 2050,1 and yet, despite more than 30 years of intensive research, we have yet to develop a drug that provides a clinically meaningful slowing in cognitive decline. There are only 6 AD drugs currently approved in the US. Five of these are symptomatic treatments, such acetylcholinesterase drugs for mild AD and the N-methyl-D-aspartate (NMDA) receptor antagonist memantine, which is used as an add-on or second-line therapy in more severe cases. Aduhelm, a beta-amyloid monoclonal antibody (mAb) therapy, represented the sixth US Food and Drug Administration (FDA) approval in AD and is the first to target the underlying pathology of the disease. However, the beta-amyloid mAbs have failed to live up to the promise of delivering a curative, disease-modifying drug (DMD). We examine directions in research and development that contribute a set of diverse pathological targets—illuminating Alzheimer’s disease as a conundrum that will likely be solved with a multifactorial treatment approach.
The Alzheimer’s Conundrum
So, why have effective AD therapeutics been so elusive? A key factor is the cavernous deficiency in our pathologic understanding of AD. In the amyloid hypothesis, plaques composed of toxic beta-amyloid and phosphorylated tau protein are hypothesized to cause neurodegeneration leading to cognitive decline. This hypothesis has dominated the last 20 years of AD research and carries with it a storm of controversy, to the extent that two camps have formed (those who support the amyloid hypothesis, and those who don’t). At issue is the failure of a long line of drugs targeting beta-amyloid in clinical trials dating back to 2003. Interviews with multiple scientists suggest that any research that fell outside of the amyloid field was suppressed,2 likely slowing progress in the field. In addition, the dire unmet need for AD therapeutics has put a strain on translational research, such that biotech companies have scrambled to move drugs into clinical trials, perhaps before the science was fully baked.
The pathology of AD, best represented as a continuum (Figure 1),1 presents another challenge. Researchers and clinicians believe that therapeutic intervention stands the best chance of success in patients with mild cognitive impairment, but without a reliable biomarker, it can be challenging to identify an appropriate patient population for clinical studies.
Figure 1. Alzheimer’s disease continuum.
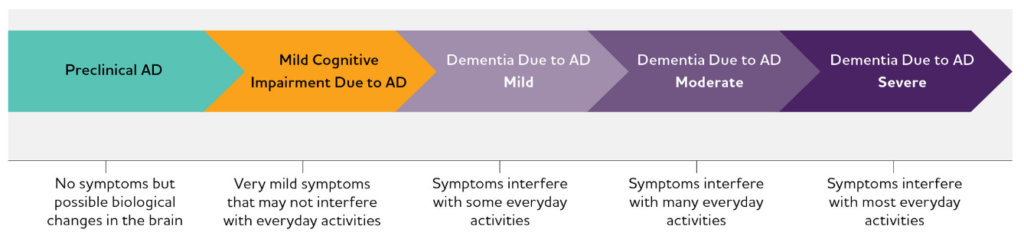
The pathological changes in the brain that cause the first noticeable symptoms of AD—related to memory, language, and cognition—are thought to start 2 decades or more prior.3-10 During this asymptomatic phase there may be measurable changes in a biomarker that could indicate future progression to clinical AD. Hence, there is a crucial need for a biomarker sensitive enough to detect AD in early stages. To date, the best available biomarker of AD is the assessment of abnormal beta-amyloid deposits in the brain by positron emission tomography (PET) imaging.
The Lumipulse® beta-amyloid test (Fujirebio Diagnostics) was FDA approved earlier this year and could potentially substitute for the use of PET scans to detect amyloid pathology; however, the test requires collection of cerebrospinal fluid (CSF), which is an unpleasant procedure. The search for a minimally invasive, blood-based biomarker has been at the center of a fervent research effort over the past decade.11 The PrecivityAD® is a blood test developed by C2N Diagnostics that has been shown to be 81% accurate in predicting the level of beta-amyloid in the brain; however, it is not yet FDA approved.
The First Drug to Address the Underlying Biology of Alzheimer’s Disease
The accelerated approval of aducanumab (Aduhelm™), an antibody that binds to and clears beta-amyloid plaques in the brain, in 2020 was a landmark in the treatment of AD, signaling the first new drug in 18 years. Aduhelm was studied in two large, randomized, controlled trials in patients with mild cognitive impairment and evidence of amyloid pathology by PET imaging. However, both trials were prematurely stopped for futility. The first trial (EMERGE) ultimately met its primary endpoint—patients on high-dose aducanumab showed a significant slowing of cognitive decline from baseline. The second trial (ENGAGE) did not meet its primary endpoint; however, patients from this trial who received sufficient exposure to high-dose aducanumab showed efficacy results supporting the findings of EMERGE. Both trials also showed a statistically significant, dose-dependent decrease in beta-amyloid and phosphorylated tau protein by PET imaging, which was the basis for accelerated approval.
Controversy ensued when an independent panel of scientific and clinical experts was assembled at an FDA Advisory Committee meeting to deliberate over the approval of Aduhelm. The committee advised unanimously against the approval of aducanumab. Despite convincing evidence that Aduhelm effectively removes beta-amyloid plaques from the brain, experts argued that the two large phase 3 clinical trials— one positive and one negative—did not conclusively demonstrate a slowing in cognitive decline.
The approval of Aduhelm has not quelled any of the controversy surrounding the amyloid hypothesis. There remains a significant unmet need for disease-modifying AD therapeutics that result in a slowing—or indeed a reversal—of cognitive decline. The following post in this Alzheimer’s series will explore the next wave of AD drug development. We will place our focus beyond aberrant amyloid and tau protein pathology, and examine a multifactorial set of disease mechanisms, including inflammatory cascades, gut-brain signaling, and axonal transport.
 Muzamil Saleem, PhD
Muzamil Saleem, PhD
Associate Scientific Director, ProEd Regulatory
Muz is a trained neuroscientist with a diverse skillset, combining a ten-year neurology-focused research career, scientific consulting experience and a three-year tenure in healthcare equity research on Wall Street before joining ProEd Regulatory—all supported by a passion for written and visual scientific communication. Connect with Muz on LinkedIn.
References
- 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18(4):700-789.
- Begley S. The maddening saga of how an Alzheimer’s ‘cabal’ thwarted progress toward a cure for decades. STAT. 2019.
- Quiroz YT, Zetterberg H, Reiman EM, et al. Plasma neurofilament light chain in the presenilin 1 E280A autosomal dominant Alzheimer’s disease kindred: a cross-sectional and longitudinal cohort study. Lancet Neurol. 2020;19(6):513-521.
- Barthelemy NR, Li Y, Joseph-Mathurin N, et al. A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer’s disease. Nat Med. 2020;26(3):398-407.
- Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357-367.
- Reiman EM, Quiroz YT, Fleisher AS, et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer’s disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 2012;11(12):1048-1056.
- Jack CR, Jr., Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132(Pt 5):1355-1365.
- Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795-804.
- Gordon BA, Blazey TM, Su Y, et al. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer’s disease: a longitudinal study. Lancet Neurol. 2018;17(3):241-250.
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70(11):960-969.
- Shi L, Baird AL, Westwood S, et al. A Decade of Blood Biomarkers for Alzheimer’s Disease Research: An Evolving Field, Improving Study Designs, and the Challenge of Replication. J Alzheimers Dis. 2018;62(3):1181-1198.
