New Weapons Emerge in the Fight Against Respiratory Syncytial Virus (RSV)
Imagine your newborn baby has been hospitalized with a serious respiratory infection and is struggling to breathe. That’s a reality for thousands of US newborns and their parents every year during the respiratory syncytial virus (RSV) season. This common endemic virus doesn’t cause clinically significant disease in most children and adults, but it can cause serious respiratory disease in some newborns—particularly those born prematurely or with underlying conditions—and for older adults with weakened immune systems.
Unfortunately, until this year there were no vaccines available to prevent RSV infection. GlaxoSmithKline (GSK) and Pfizer both made history earlier this year with the approval of an RSV vaccine for older adults. On May 3, the US Food and Drug Administration (FDA) approved GSK’s Arexvy (the world’s first RSV vaccine for older adults), and on May 31, FDA approved Pfizer’s ABRYSVO™ RSV vaccine. In both cases, these vaccines were shown to significantly prevent lower respiratory tract infection caused by RSV in individuals 60 years of age or older. But what about infants and young children at risk for serious disease?
Every year in the United States nearly 600,000 infants and young children—mostly healthy, full‑term infants in their first year of life—will develop a lower respiratory tract infection from RSV, and up to 80,000 will be hospitalized.1,2 Some of these infants will require intensive care with oxygen and intravenous (IV) fluids, and a few of the most vulnerable infants—those born prematurely with underdeveloped lungs or with congenital heart disease—may die.
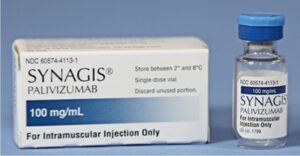
Until now, the only option to prevent RSV infection was an anti-RSV antibody developed by MedImmune in the 1990s known as Synagis®. Synagis® (palivizumab) was approved in 1998, but only for use in high-risk infants like those described above. Synagis can be given during the 5-month RSV season, and it neutralizes the virus, thus reducing the risk of infection and serious respiratory disease. However, it requires 5 monthly intramuscular injections, and there was nothing available to prevent RSV infections in healthy, full-term infants.
But that all changed this week with the FDA approval of BEYFORTUS™ (nirsevimab) on July 17, which is indicated for the prevention of RSV lower respiratory tract disease in neonates and infants born during or entering their first RSV season, and in children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season. Nirsevimab is a potent RSV‑neutralizing antibody that can be given to an infant before or during the RSV season, and it has been engineered to have an extended half-life.
There will soon be two new weapons available in the US to protect infants and young children from RSV infection. The first is nirsevimab, which has been shown to provide ~75% protection from any medically attended RSV-associated lower respiratory tract infection for at least 5 months. Thus, a single shot can protect the infant for the entire RSV season. On June 8th, the Antimicrobial Drugs Advisory Committee unanimously endorsed approval of nirsevimab for all neonates and infants born during or entering their first RSV season. Importantly, there are no serious safety concerns with nirsevimab. A small number of infants who received the antibody had a minor rash or injection site reaction, as would be expected with a childhood vaccination, but there was no evidence of any serious risks associated with nirsevimab.
There will soon be two new weapons available in the US to protect infants and young children from RSV infection.
The second is a vaccine that can be given to the mother with the goal of boosting maternal antibodies against the virus, which are passed to the fetus through the placenta. Pfizer’s maternal vaccine ABRYSVO™ (the same formulation as the adult vaccine described above) was reviewed by the FDA Vaccines and Related Products Advisory Committee on May 18th and received a favorable recommendation. The maternal vaccine can be administered during the 2nd or 3rd trimester and provides the baby with about 80% protection from severe RSV lower respiratory tract infection for 3 to 4 months after birth. The advisory committee expressed some concern about the potential for the vaccine to increase the number of premature births but ultimately agreed that the benefits outweigh the risks. FDA will make a decision about the Pfizer vaccine in the near future.
Nirsevimab has the added benefit that it can provide protection regardless of when the baby is born. Nirsevimab can be given to the infant in the hospital, immediately after birth, or by a pediatrician just before the RSV season begins. That’s important because the RSV season in the United States typically occurs from October to March in most locations. So, if a mother who received the maternal vaccine gives birth in the Spring, her baby will no longer be protected when the next RSV season begins in October because of the waning of maternal antibodies present in the baby after 3 to 4 months. In such a case, the baby can be given nirsevimab in September or October and be protected for the full RSV season.
Together, these two interventions could dramatically reduce the burden of RSV infection in the United States. It is estimated that if every infant born in the United States received nirsevimab, it could prevent 300,000 RSV-related medical visits, 100,000 emergency room visits, and up to 60,000 hospitalizations every year. That would have a huge impact on busy pediatric emergency rooms and intensive care units that are often overwhelmed during the winter months due to RSV and influenza.
Together, these two interventions could dramatically reduce the burden of RSV infection in the United States.
The Long Road to RSV Prevention
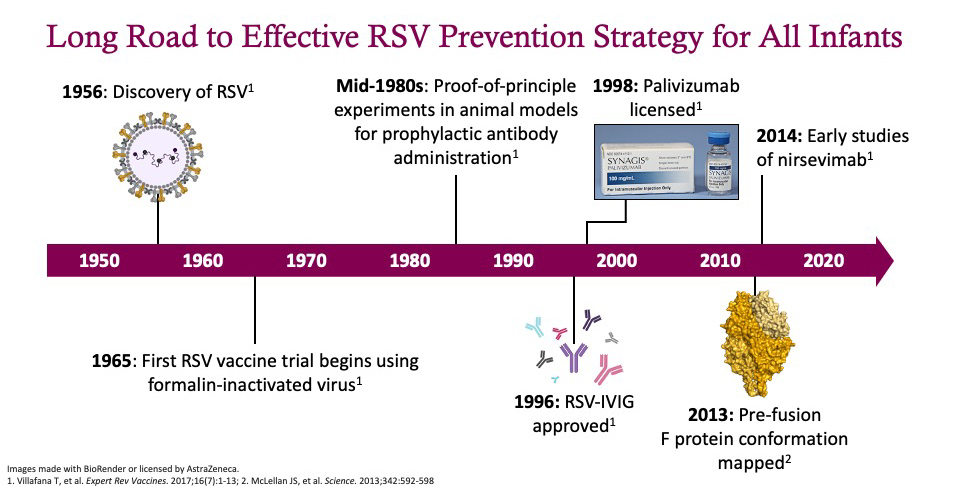
It has been a long road to developing a broadly effective RSV-prevention strategy.3 The virus was first discovered in the 1950s, and the first trials of a formalin‑inactivated vaccine were conducted in the mid-1960s. However, that vaccine caused enhanced disease in some children who were later exposed to RSV and resulted in the death of two infants, which dramatically impeded subsequent vaccine development.
In the mid-1980s, the first studies demonstrating passive immunization with an antibody were completed; this led to the development of RSV-intravenous immunoglobulin (IVIG) and its approval in 1996. Next came the approval of palivizumab in 1998, an antibody that targets the RSV F fusion protein.
In 2013, the conformational mapping of the prefusion F protein by Jason McLellan and Barney Graham at the National Institutes of Health revolutionized the field.4 They identified important epitopes on the prefusion F protein, including Site 0, which is highly conserved and when targeted by antibodies can neutralize the virus so that it cannot infect cells. This ground-breaking research ultimately led to development of nirsevimab in 2014.
The FDA approval of both vaccines and neutralizing antibodies against RSV in 2023 is another shining example of the power of biomedical research to address infectious diseases that pose a threat to human health. Although it took much longer to achieve this goal than anyone could have anticipated when the virus was first identified in 1958, science ultimately prevailed, thanks to the thousands of parents who were willing to enroll in clinical trials.
The FDA approval of both vaccines and neutralizing antibodies against RSV in 2023 is another shining example of the power of biomedical research to address infectious diseases that pose a threat to human health.
References
- Rainisch G, Adhikari B, Meltzer MI, Langley G. Estimating the impact of multiple immunization products on medically attended respiratory syncytial virus (RSV) infections in infants. Vaccine. 2020;38(2):251-257.
- McLaughlin JM, Khan F, Schmitt H-J, et al. Respiratory syncytial virus-associated hospitalization rates among US infants: a systematic review and meta-analysis. J Infect Dis. 2022;225(6):1100-1111.
- Villafana T, Falloon J, Griffin MP, et al. Passive and active immunization against respiratory syncytial virus for the young and old. Expert Rev Vaccines. 2017;16(7):1-13.
- McLellan JS, Chen M, Joyce MG, et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science. 2013;342:592-598.

Jeffrey S. Riegel, PhD
SVP, Scientific Communications, ProEd Regulatory
Jeff combines his scientific expertise in molecular biology and immunology with more than 25 years of global healthcare agency experience in guiding medical and regulatory communication strategies for biopharma companies. Jeff leads the scientific team at ProEd Regulatory, which helps clients prepare for FDA Advisory Committee meetings and other health authority interactions. Connect with Jeff on LinkedIn.